Planmed Verity Extremity Scanner receives Health Canada Medical Device License
Press release
Planmed is pleased to announce that the company has received the Health Canada approval for its Planmed Verity® Extremity Scanner. Planmed Verity is intended to be used for X-ray cone-beam computed tomography of anatomies within upper and lower extremities.
“We are excited that this innovative, low dose orthopedic imaging system is now available also in Canada. Planmed Verity is the first and only CT system in the world that can image seated, supine and standing patients. The US FDA gave its clearance for the device already earlier this year,” says Vesa Mattila, Managing Director of Planmed Oy.
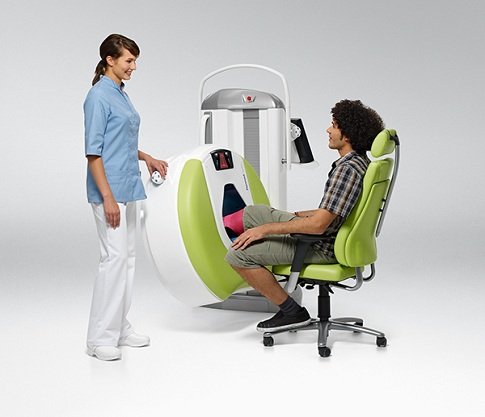
Planmed Verity® is designed to find subtle extremity fractures during the first visit to the clinic – the types of fractures that have most commonly been missed when using only 2D radiographs. Planmed Verity Extremity Scanner is a unique solution to the problem; it provides fast 3D imaging at the point of care. It is intended for pre- and postoperative imaging; and it has better resolution and patient adaptability and uses a significantly lower dose of radiation than full-body CTs. Unlike any other 3D imaging device, Planmed Verity also allows for weight-bearing imaging of the extremities.
As a dedicated extremity scanner, Planmed Verity adapts to the patient with anatomy-specific imaging programs, movements, and trays. Easily adjustable, soft-surfaced gantry and motorized positioning trays help in finding a comfortable position for various examination procedures. The adjustable user interface and efficient all-in-one workflow are also designed to maximize the operator’s soothing presence for the patient.
Planmed’s products are well known for their exquisite design and user ergonomics. The U.S. subsidiary Planmed USA, Inc. located in Roselle, IL, is responsible for sales, marketing, and tech support for Planmed products in USA and Canada. “Planmed has been active with analog and digital mammography in Canada. We are very pleased to extend our imaging equipment line to orthopedic imaging there,” says Chris Oldham, Director of Sales of Planmed USA, Inc.
Planmed Verity received the US FDA clearance in February and already has the CE mark, South Korean KFDA approval, Russian registration certification and Australian TGA listing.
Planmed Oy and the Planmeca Group
Planmed Oy develops, manufactures, and markets advanced imaging equipment and accessories for mammography and orthopedic imaging. Planmed's extensive mammography product range covers digital and analog units, stereotactic biopsy devices, and breast positioning systems for an early detection of breast cancer. Within the field of orthopedic 3D imaging, Planmed offers low-dose extremity CT imaging for quicker, easier and more accurate diagnosis at the point of care. Planmed Oy exports more than 98% of its production to more than 70 countries worldwide. The principal markets are in Europe, Japan, and Oceania, as well as in North and Latin America, where the company has considerable market shares.
Planmed Oy is part of the Finland-based Planmeca Group, which manufactures and markets advanced equipment for medical and dental fields. The Group employs approximately 2,500 professionals and the estimated turnover for the year 2013 is MEUR 760.
www.planmed.com